Introduction to Stem Cells in Medicine
Imagine having cells in your body that could turn into any other type of cell - brain cells, heart cells, or even skin cells. These amazing cells exist and they're called stem cells! They're like the body's own repair kit and scientists are finding incredible ways to use them to treat diseases and injuries that were once thought impossible to cure.
Key Definitions:
- Stem Cell: A special type of cell that can divide and develop into many different types of specialised cells.
- Differentiation: The process where a stem cell becomes a specific type of cell with a particular function.
- Pluripotent: Able to develop into almost any type of cell in the body.
- Totipotent: Able to develop into any type of cell, including cells that form the placenta.
🔬 What Makes Stem Cells Special?
Stem cells have two unique abilities that make them incredibly valuable in medicine. First, they can make exact copies of themselves through cell division. Second, they can transform into specialised cells like muscle cells, nerve cells, or blood cells. Think of them as the ultimate shape-shifters of the biological world!
Types of Stem Cells
Not all stem cells are the same. Scientists have identified several different types, each with their own special abilities and potential uses in medicine.
Embryonic Stem Cells
These are the most powerful stem cells and come from very early embryos (about 3-5 days old). They're pluripotent, meaning they can become almost any type of cell in the human body. However, their use is controversial because obtaining them involves destroying embryos.
🌱 Advantages
Can become any cell type, unlimited supply through lab cultivation, very effective for research and potential treatments.
⚠ Challenges
Ethical concerns about embryo use, risk of immune rejection, potential for tumour formation.
🔬 Current Use
Mainly used in research to understand diseases and test new treatments, limited clinical trials.
Adult Stem Cells
These stem cells are found in various parts of the adult body, including bone marrow, fat tissue and blood. They're more limited than embryonic stem cells but are already being used successfully in treatments. The most famous example is bone marrow transplants for leukaemia patients.
Real-World Success Story
In 2019, a patient with sickle cell disease received a bone marrow transplant using stem cells from a matched donor. The stem cells produced healthy red blood cells, effectively curing the patient of this painful genetic condition. This shows how adult stem cells are already saving lives today!
Stem Cells in Medical Treatment
The medical applications of stem cells are expanding rapidly. From treating blood disorders to potentially curing paralysis, stem cells offer hope for millions of patients worldwide.
🩸 Blood Disorders
Bone marrow transplants using stem cells have been treating leukaemia, lymphoma and other blood cancers for decades. The stem cells replace diseased blood-forming cells with healthy ones, giving patients a new lease on life.
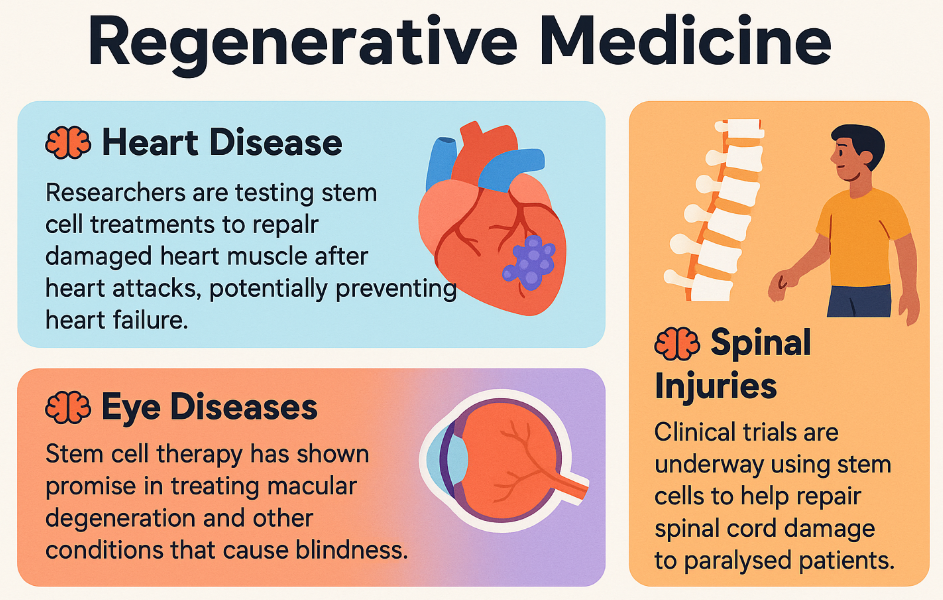
Regenerative Medicine
This is where stem cells really shine. Scientists are working on growing new organs and tissues to replace damaged ones. Imagine being able to grow a new heart for someone with heart disease or new spinal cord tissue for someone who's paralysed!
Case Study: Parkinson's Disease Treatment
In 2020, researchers in Japan began a groundbreaking trial using induced pluripotent stem cells (iPSCs) to treat Parkinson's disease. They converted skin cells from patients into stem cells, then into dopamine-producing brain cells. These cells were then transplanted back into the patients' brains. Early results suggest the treatment may help reduce symptoms and slow disease progression.
Induced Pluripotent Stem Cells (iPSCs)
One of the most exciting developments in stem cell research is the creation of iPSCs. These are adult cells that have been reprogrammed to act like embryonic stem cells. This breakthrough won the Nobel Prize in 2012!
How iPSCs Work
Scientists take ordinary adult cells (like skin cells) and add special proteins that turn back the clock, making them behave like embryonic stem cells again. It's like giving cells a fountain of youth!
🎉 Benefits of iPSCs
No ethical issues since no embryos are used, reduced risk of immune rejection since cells come from the patient and unlimited potential for personalised medicine.
Challenges and Limitations
While stem cell therapy holds enormous promise, there are still significant hurdles to overcome before it becomes widely available.
Scientific Challenges
Controlling how stem cells develop is incredibly complex. Scientists need to ensure cells become the right type and don't form tumours. It's like trying to conduct an orchestra where every instrument needs to play perfectly in harmony.
⚠ Safety Concerns
Risk of tumour formation, immune rejection and unexpected side effects require extensive testing.
💰 Cost Issues
Developing and producing stem cell treatments is extremely expensive, making them potentially inaccessible to many patients.
📈 Technical Hurdles
Controlling cell differentiation, ensuring quality and consistency and scaling up production remain major challenges.
Ethical Considerations
The use of stem cells, particularly embryonic stem cells, raises important ethical questions that society continues to debate.
The Ethical Debate
The main ethical concern centres around embryonic stem cell research. Some people believe that embryos, even at very early stages, deserve protection and shouldn't be destroyed for research. Others argue that the potential to save millions of lives justifies this research. This debate has led to different regulations in different countries and has pushed scientists to develop alternatives like iPSCs.
Balancing Benefits and Concerns
Most countries have developed guidelines that try to balance the potential benefits of stem cell research with ethical concerns. These often include strict oversight of research, informed consent from donors and restrictions on certain types of research.
The Future of Stem Cell Medicine
The future looks incredibly bright for stem cell medicine. Scientists are working on treatments for conditions that currently have no cure, from Alzheimer's disease to diabetes.
🚀 Emerging Applications
3D bioprinting of organs, personalised drug testing, treatment of genetic diseases and even potential anti-ageing therapies are all on the horizon.
As our understanding of stem cells grows and technology advances, we're moving closer to a future where many currently incurable diseases could become treatable. The key is continued research, careful regulation and public support for this revolutionary field of medicine.